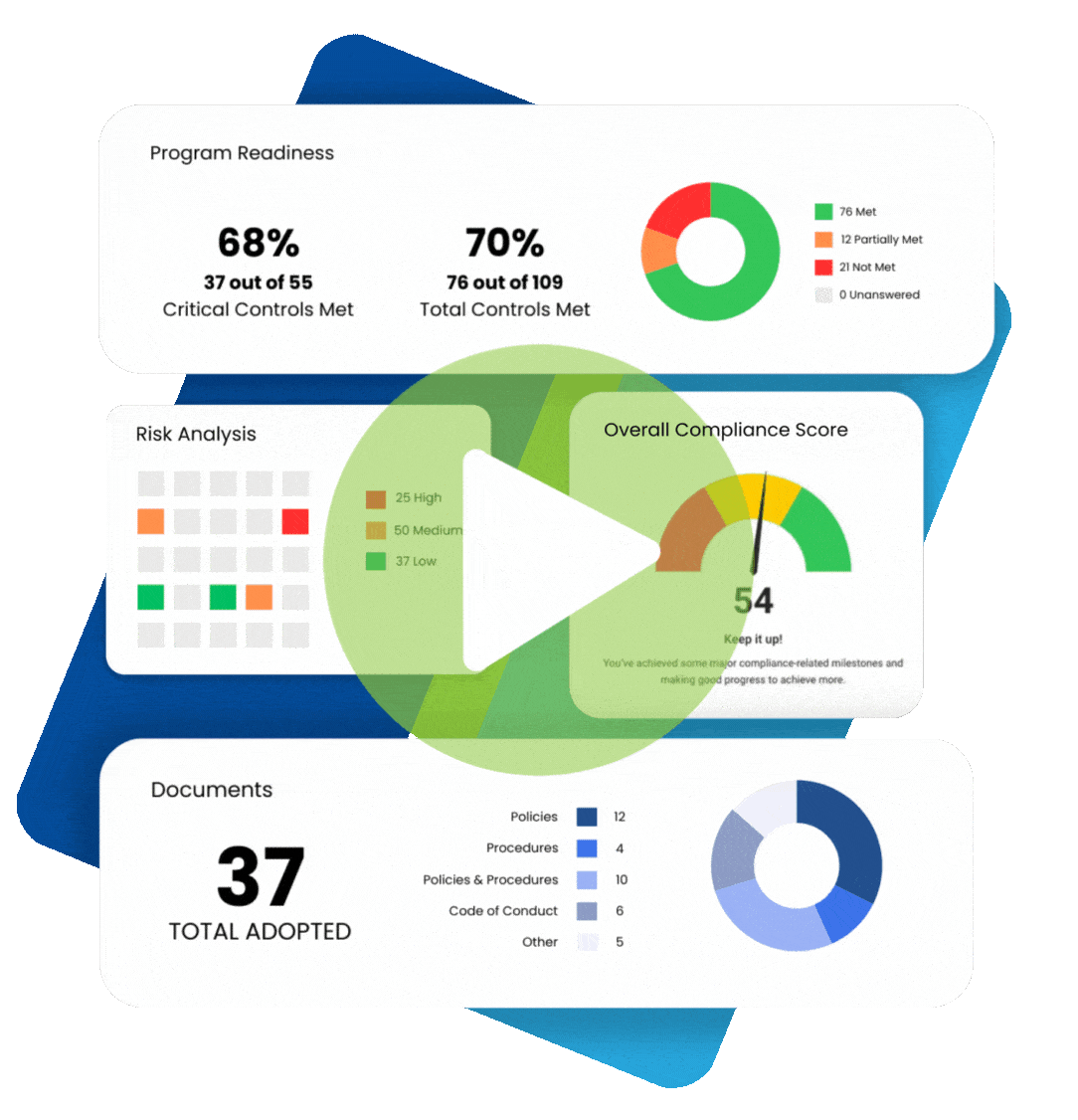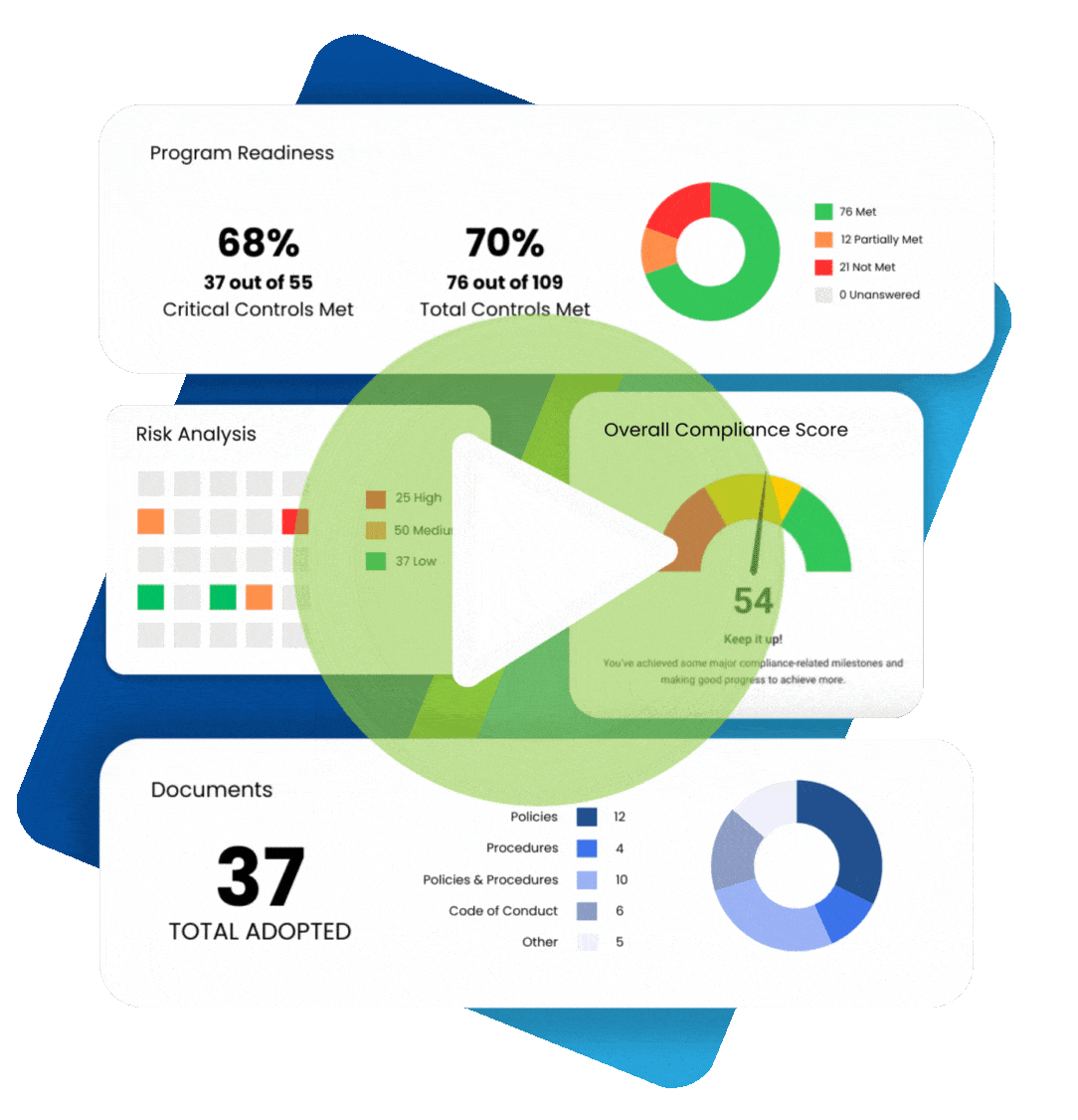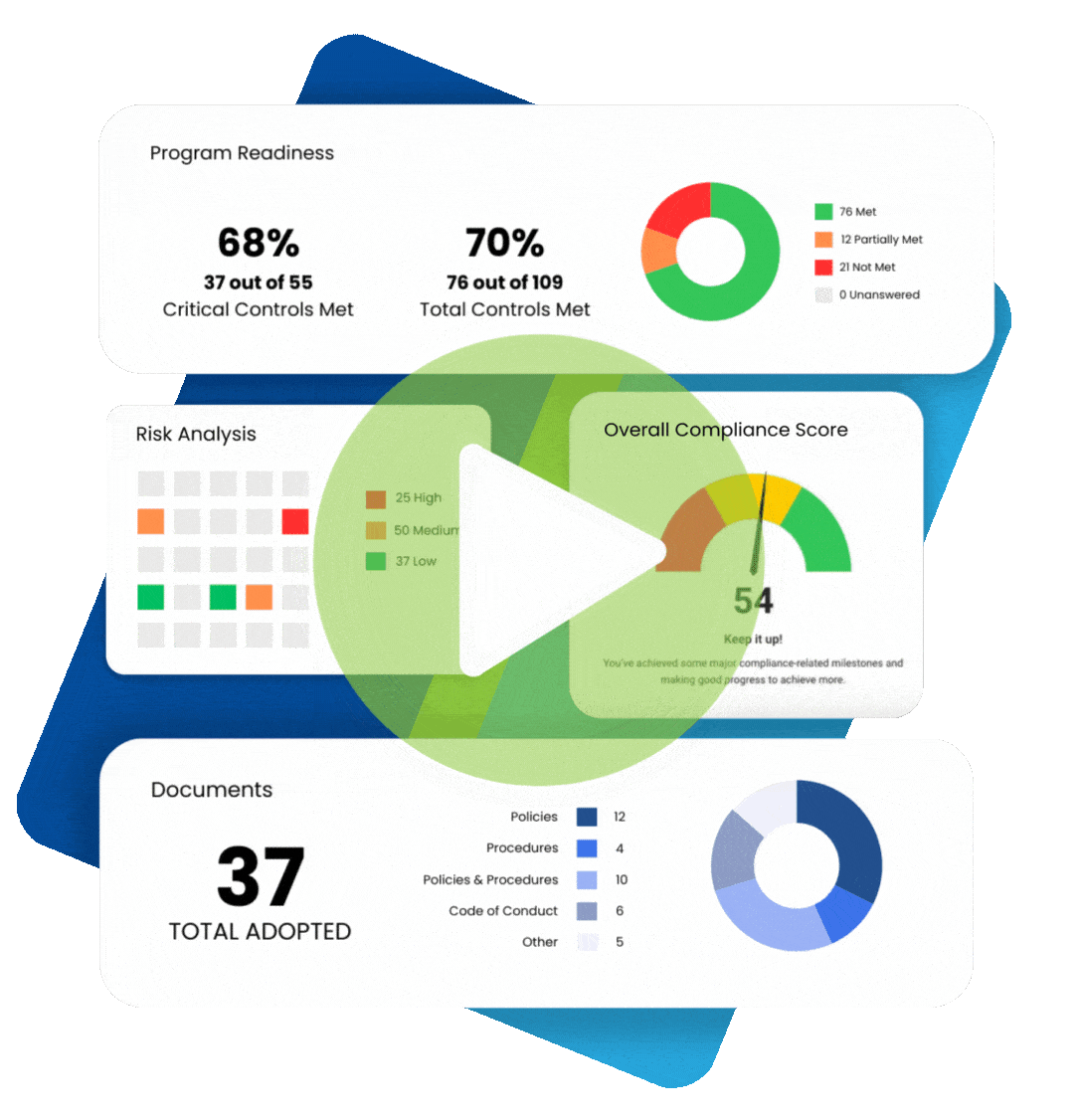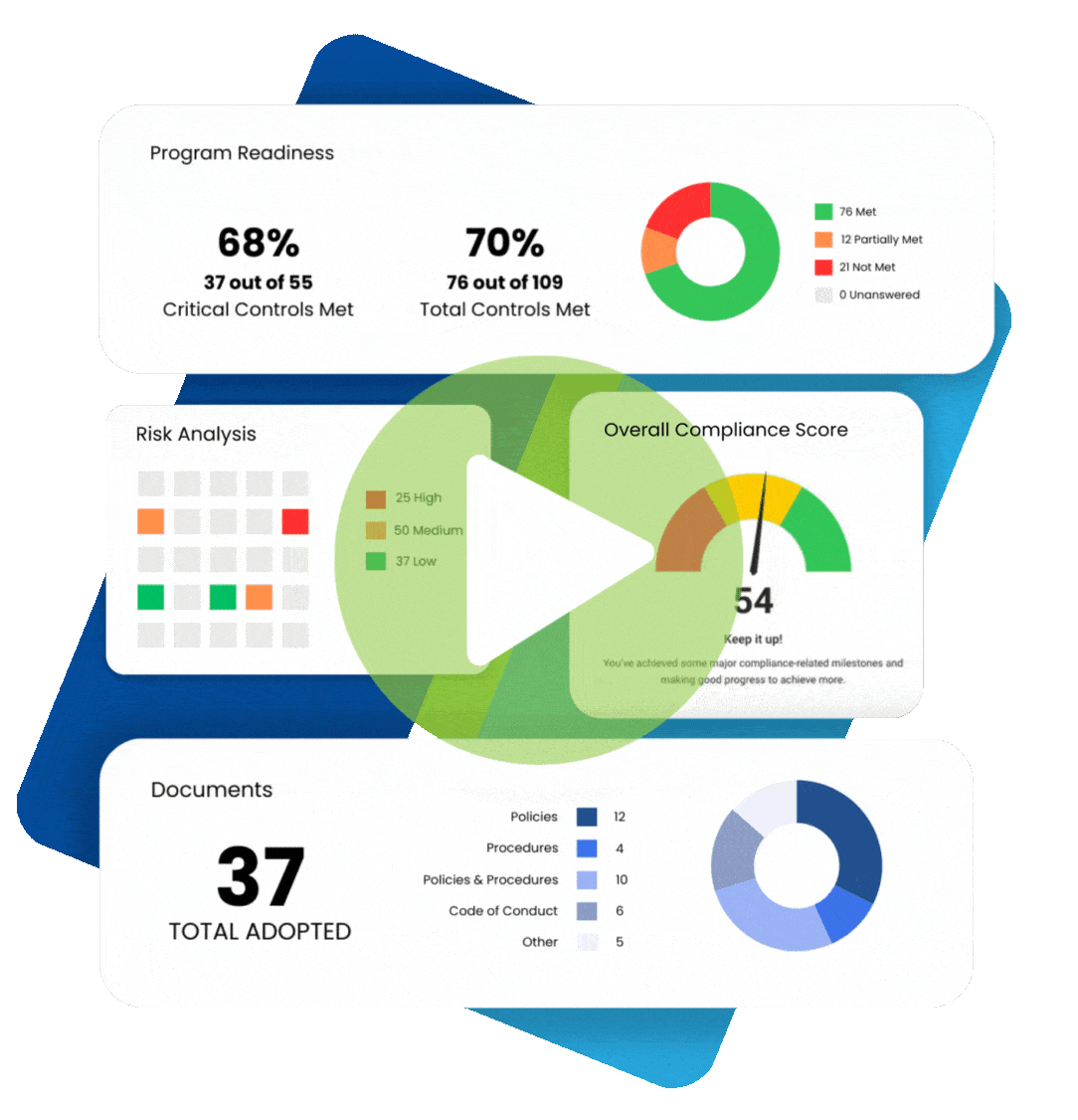
Maintaining healthcare compliance depends on having many tools and strategies at your disposal, especially when faced with an audit or incident of non-compliance. Perhaps the most essential element is compliance documentation. Paperwork can be a chore, but these documents help you keep track of all the moving parts that make up regulatory healthcare compliance.
From safeguarding protected health information (PHI) to conducting risk assessments, compliance documents help your organization mitigate risk and stay in the good graces of governmental agencies. We examine the nature of compliance documentation, its importance, and how you can maintain a solid documentation framework.
What Does Compliance Documentation Involve?
Compliance documentation refers to collecting, sharing, maintaining, and storing reports and records that enable healthcare organizations to adhere to various healthcare regulations. Compliance documents help you perform multiple functions:
- Prove adherence to a law, especially during audits
- Record a compliance violation
- Collect data and report analysis results
- Perform an audit
- Document the results of a risk assessment
- Provide a detailed response plan after an incident
- Document corrective actions an organization takes after a reported violation
- Keep records of training and other compliance-related activities
- Provide compliance-related guidance to staff and publicize standard operating procedures (SOPs)
- Facilitate and document internal and external communication regarding compliance activities
- Inform staff and stakeholders of legislative changes
Keeping accurate and comprehensive records allows you and other compliance professionals to fulfill your roles and meet your organization’s compliance needs.
Importance of Compliance Documents to Your Healthcare Organization
Besides enabling various compliance activities, readily available compliance documents can make a significant difference in maintaining your entity’s reputation. In the pursuit of minimizing fraud, waste, and abuse, keeping careful records helps you and your organization:
- Avoid civil lawsuits and heavy fines for non-compliance
- Stay informed about regulatory changes
- Ensure that all employees have equal access to critical information
- Prevent employees from letting their compliance training lapse
- Show transparency in compliance activities
- Identify security gaps and other risks
- Make compliance audits go more smoothly
Developing a Compliance Documentation Framework
Compliance officers should adopt an organizing framework that guides the collection, sharing, and storage of compliance documents. If you work for a healthcare organization, you’ll typically develop a framework reflecting the norms established by regulations like the Health Insurance Portability and Accountability Act (HIPAA).
Within your framework, your compliance documents should include information and guidance on the following:
- Data collection policies: Patient and customer access to PHI, protection of sensitive data.
- Operational policies and procedures: Data collection and privacy, ethical and legal practices, audit and risk assessment procedures, and best practices for operational efficiency.
- Plans for recovery and remediation: Protocols for reporting incidents and implementing corrective actions, obtaining plan approval from organizational leaders, and documenting updates to current plans.
Types of Compliance Documents to Maintain and Report
When thinking about compliance documentation, it’s easy to imagine the busy shuffling of paperwork — real or virtual. However, understanding the different types of documents to maintain compliance is easier if you think of them as falling into one of four categories.
Operational documents detail the internal policies and procedures staff must follow and use to carry out their duties in compliant ways. These documents include:
- Crisis management and response plans
- Onboarding and other human resources (HR) documentation
- Employee codes of conduct
- Vendor and third-party agreements
Data privacy documents refer to security measures and records detailing data and PHI management, financial records, intellectual property, and other protected information. Examples include confidentiality agreements, business associate agreements, copyrights, and patents.
Technical security documents include reports that help compliance officers and other personnel detect data risks and make necessary changes to procedures and network infrastructure elements. This document category comprises surveillance, backup, update log, and maintenance records.
Finally, audit documents refer to reports on the organization’s past and ongoing compliance audits. These compliance documents include:
- Risk assessment reports
- Remediation plans
- Results of vulnerability scans part of audits
Support From Compliance Document Services
Most compliance officers find it time-consuming to keep track of all the required compliance documents. Moreover, even the most experienced compliance professionals could benefit from more clarification on the entire process.
That’s why you and your organization can get help from compliance document service providers. Compliancy Group offers compliance software packages that provide templates for risk assessments, procedures, policies, and other essential documents. Our software also provides a centralized platform to manage, share, and store all records related to your compliance activities.