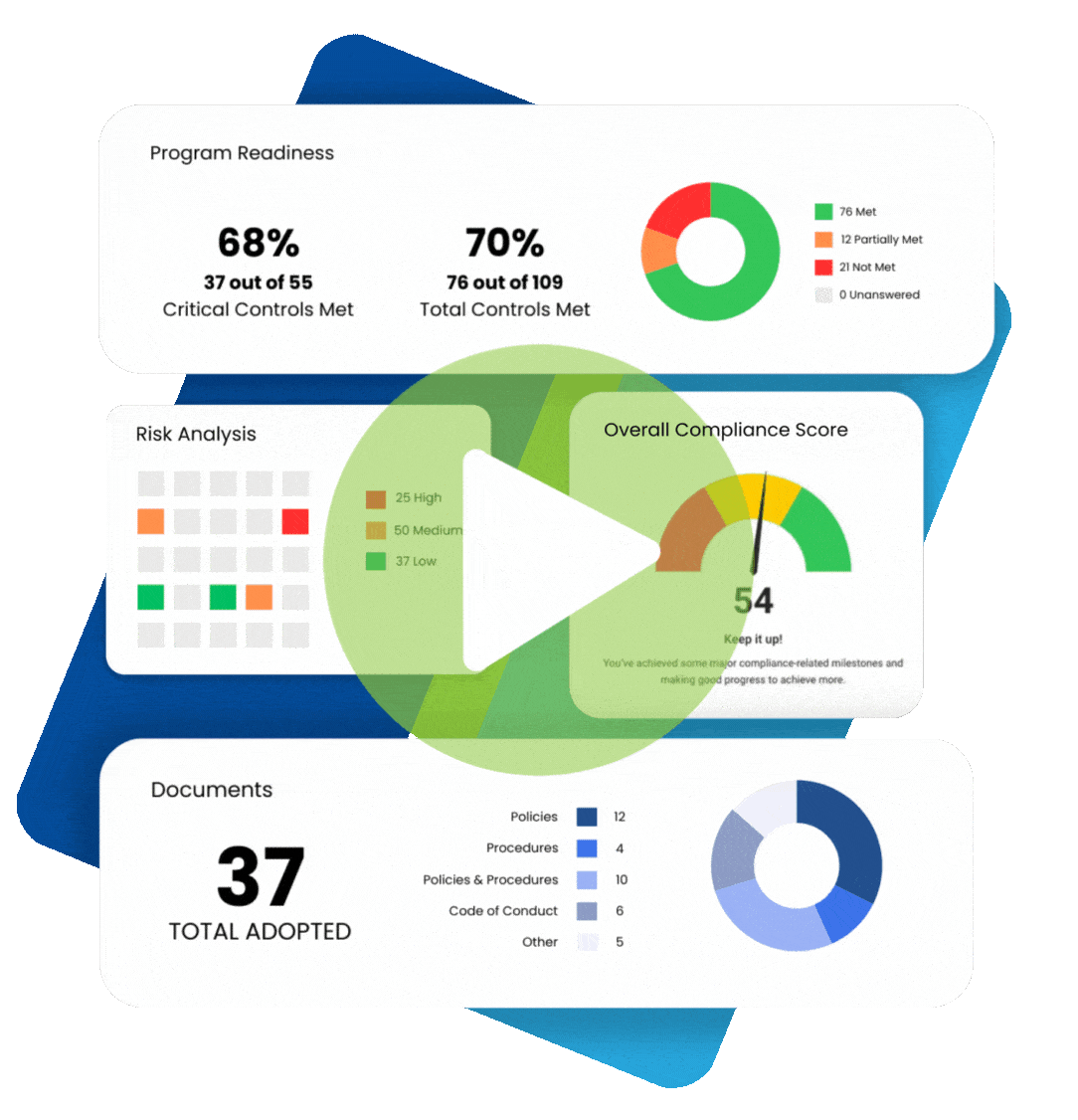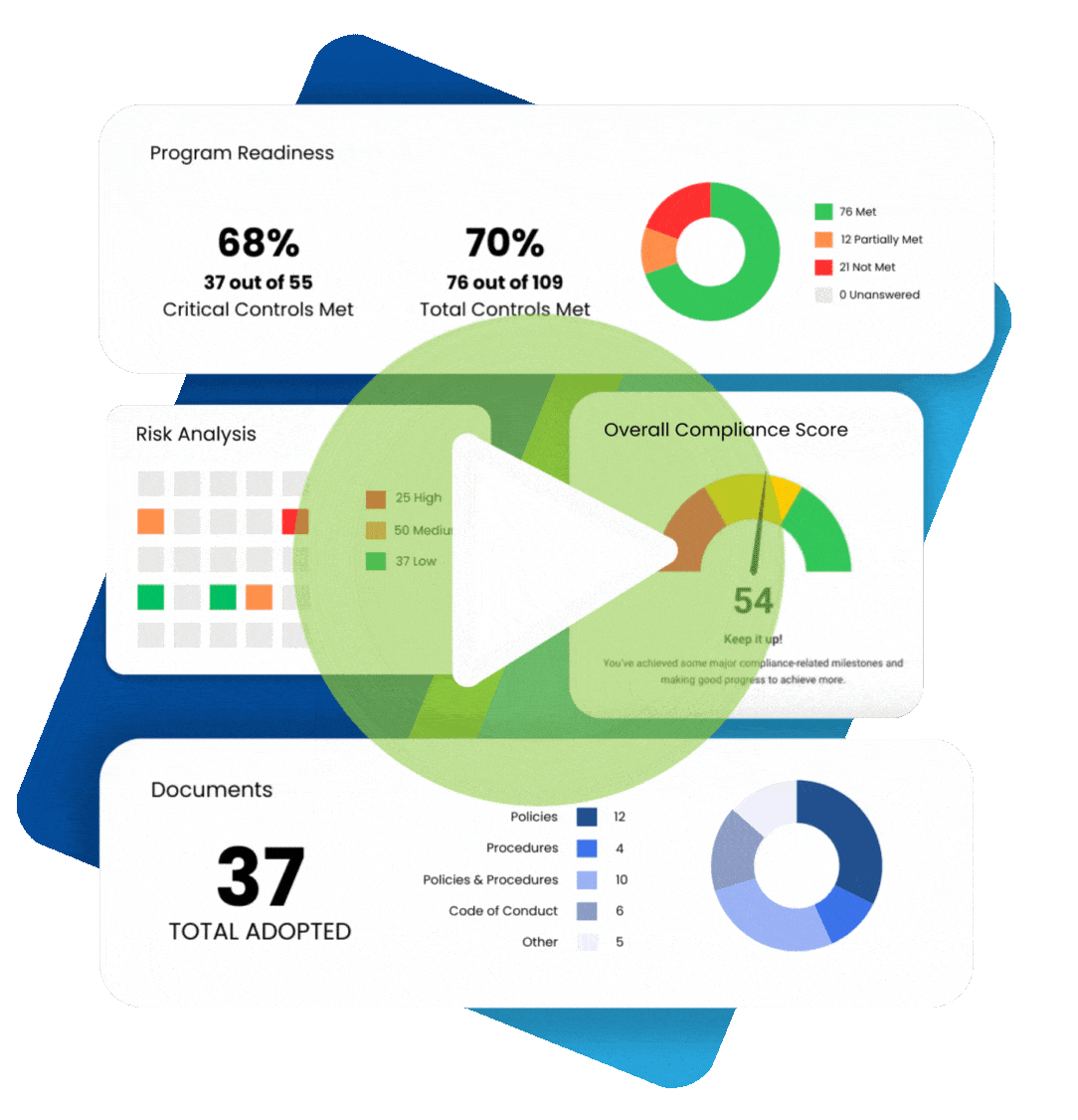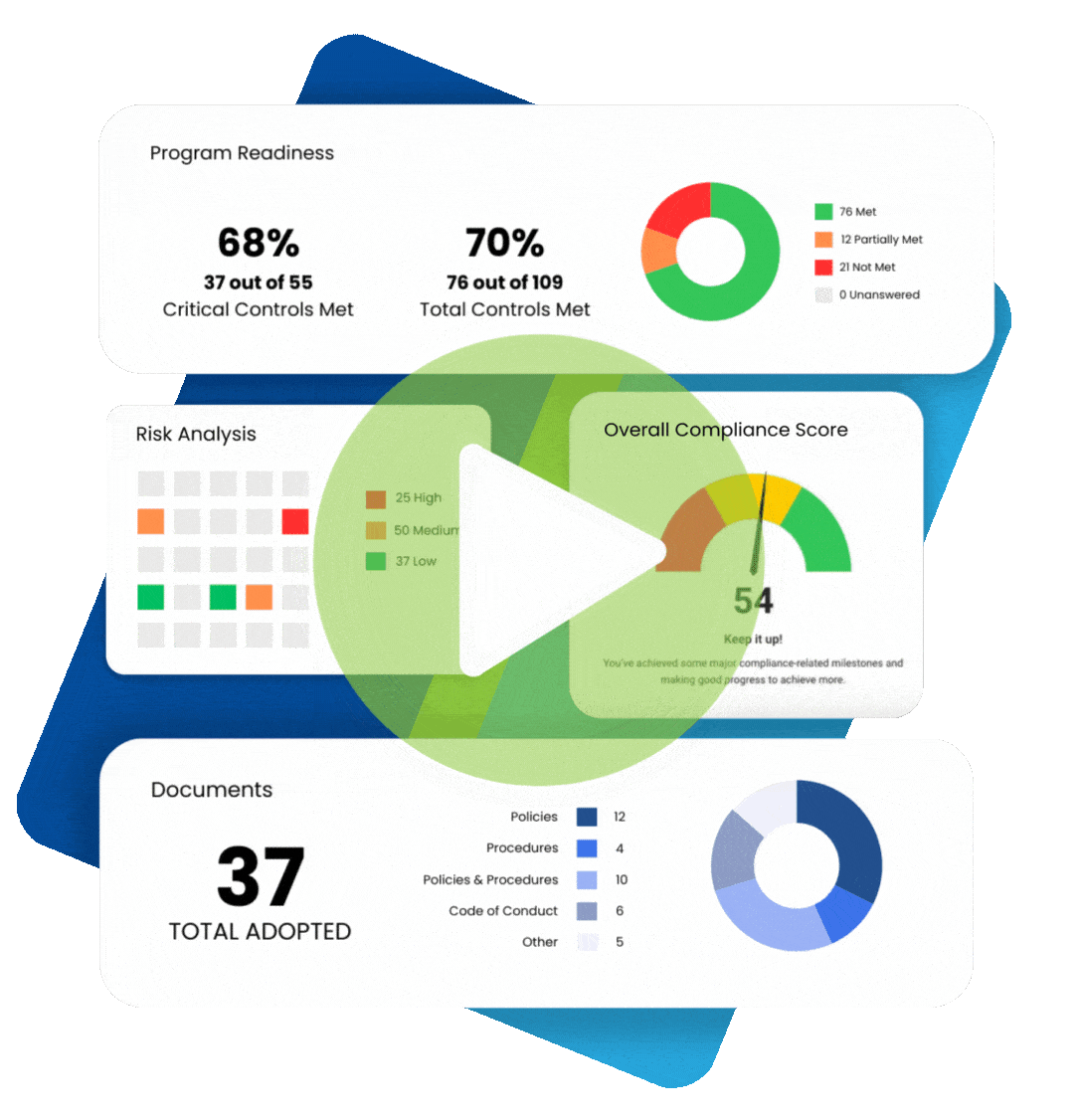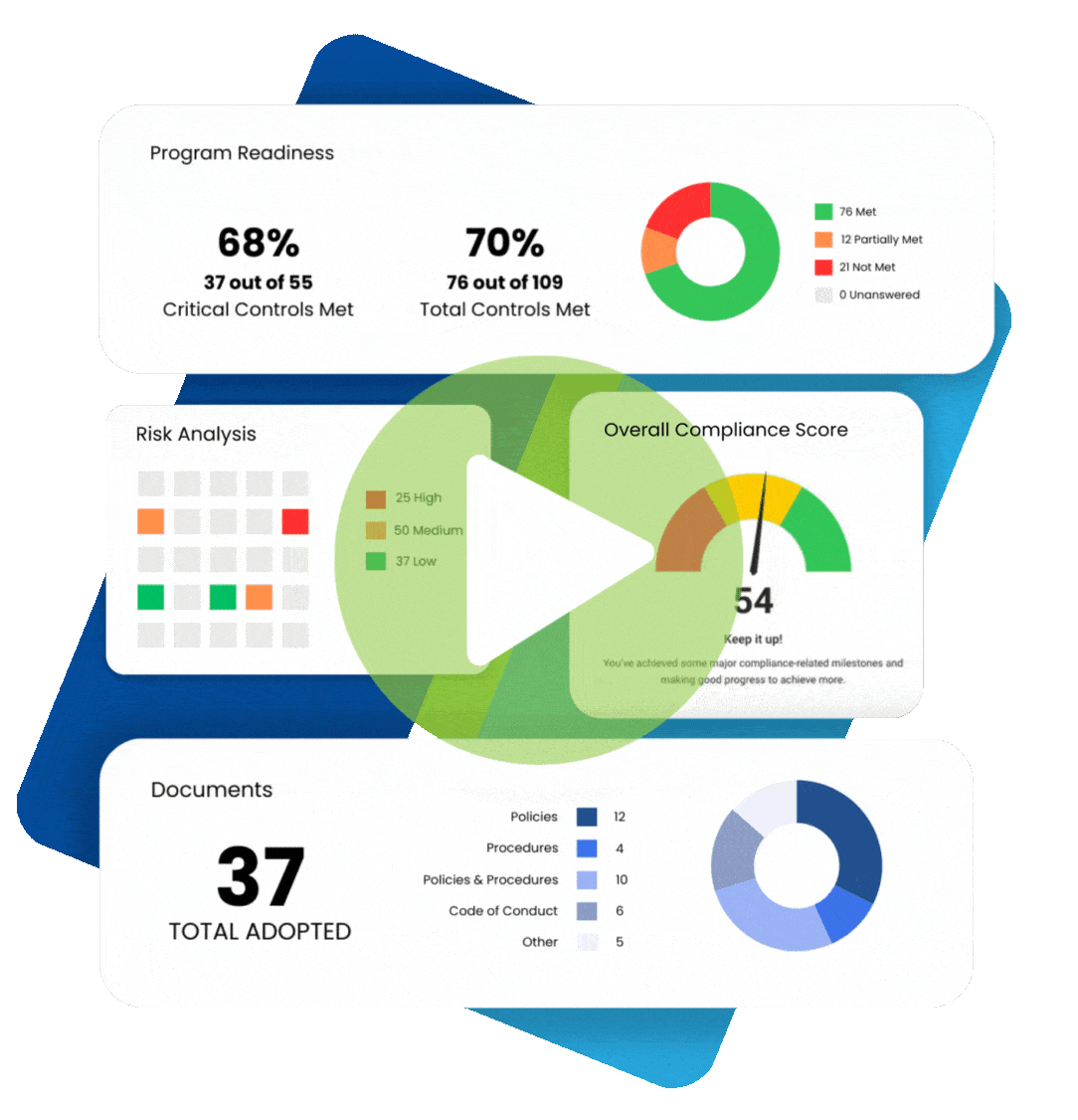
When understanding what practices are permissible under the Health Insurance Portability and Accountability Act (HIPAA), it makes sense to plan for various contingencies. For example, if a patient cannot provide written consent for releasing their protected health information (PHI), is verbal consent permitted for HIPAA? The answer is “yes” under certain circumstances. We review the fundamentals of HIPAA informed consent and discuss situations allowing HIPAA verbal consent to release information.
The Law Behind HIPAA Informed Consent
As the HIPAA Privacy Rule outlines, informed consent helps prevent the unauthorized use and disclosure of PHI. Informed consent requires healthcare providers to obtain patients’ written authorization to release their information to other parties, such as another doctor, as part of their healthcare. Informed consent also includes letting patients know all the risks and benefits associated with allowing for the release of their sensitive information.
A signed consent form authorizing the release of PHI must contain the following:
- The patient’s PHI to be used and disclosed
- The person who will be authorized to use or disclose the PHI and individuals or entities potentially receiving the disclosed PHI
- An expiration date for the authorization
Typically, the patient reads the informed consent form in print or digital form. If they consent, they sign the form, indicating that they understand the terms and provide authorization for releasing their PHI.
When HIPAA Verbal Consent to Release Information Is Allowed
In some situations, it’s not possible or necessary to obtain a patient’s written informed consent. The following circumstances allow for HIPAA verbal consent to release information:
- Inclusion of information in a hospital directory: When a patient is admitted to the hospital, they can authorize public access to their name, location, and health condition. This option might be desirable for patients with family members wanting to stay informed of their conditions or treatments. Consent can be verbal or in writing.
- Updates for family or friends: Patients can bypass the paperwork and verbally consent to their provider to give abbreviated notifications to close family members and caregivers. This option is available in case patients are unable to communicate their preferences.
- Human subjects research with minimal risks: For most clinical studies, written informed consent from human subjects is required. However, if the participation risks are minor, a prospective subject can waive written consent. HIPAA verbal consent to release information still requires detailed documentation.
Providers obtaining verbal consent must create a written record that includes the date and time, the name of the person giving informed consent, and details regarding the consent. Documenting each instance of HIPAA verbal consent to release information provides proof if accusations of privacy violations or other issues emerge later.
Software Support for HIPAA Informed Consent Issues
Obtaining informed consent from patients requires detailed knowledge of what to do in unique circumstances, such as when patients can’t provide written consent. Compliancy Group offers solutions that keep you in the know regarding HIPAA privacy regulations. Our software can streamline your recordkeeping and provide templates for creating policies regarding the use and disclosure of PHI.
On occasion, your healthcare providers likely get asked, “Is verbal consent permitted for HIPAA?” With software and guidance from Compliancy Group, your team can answer this and other questions with confidence. Contact us today for more information about our software and other services.