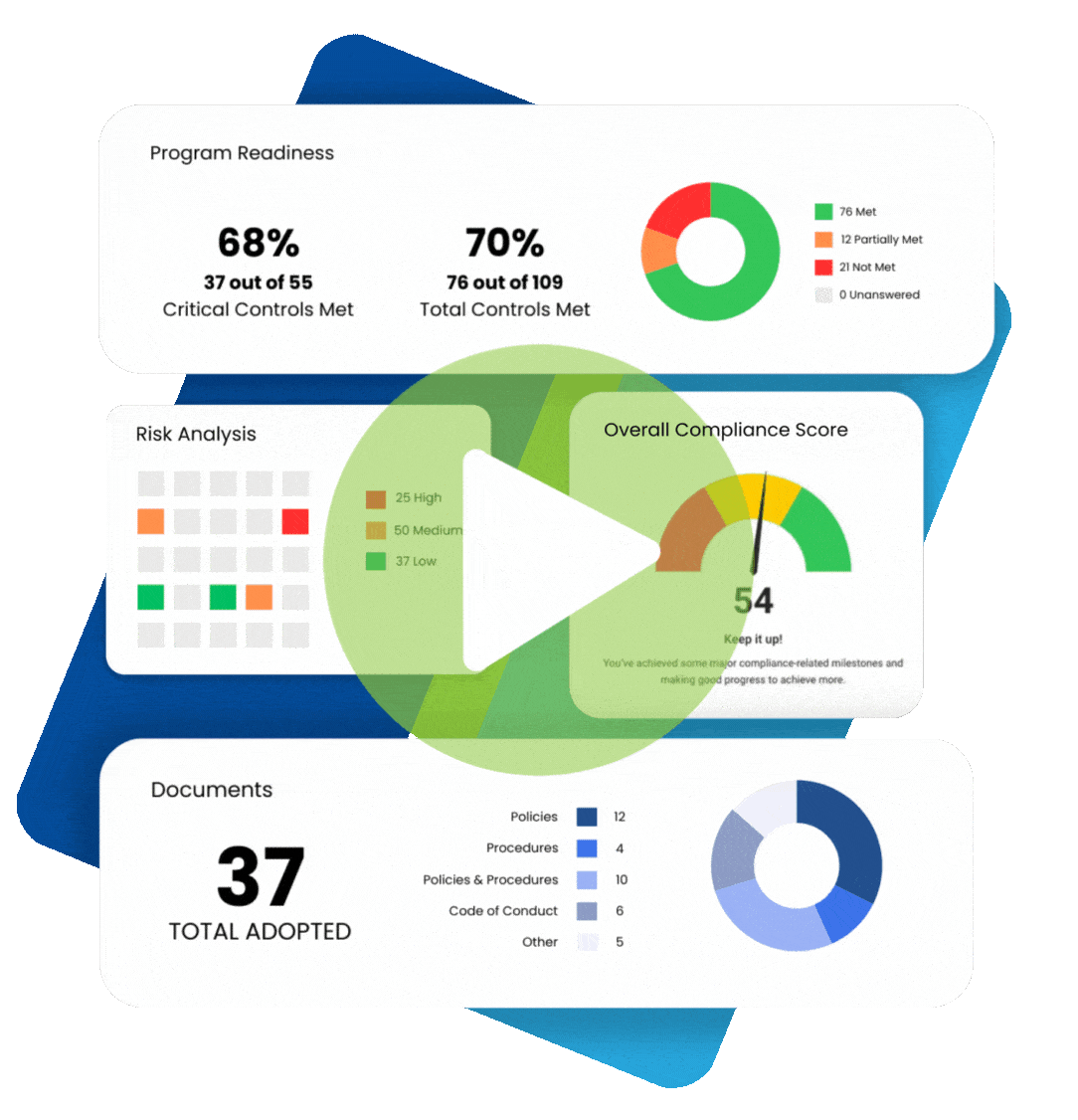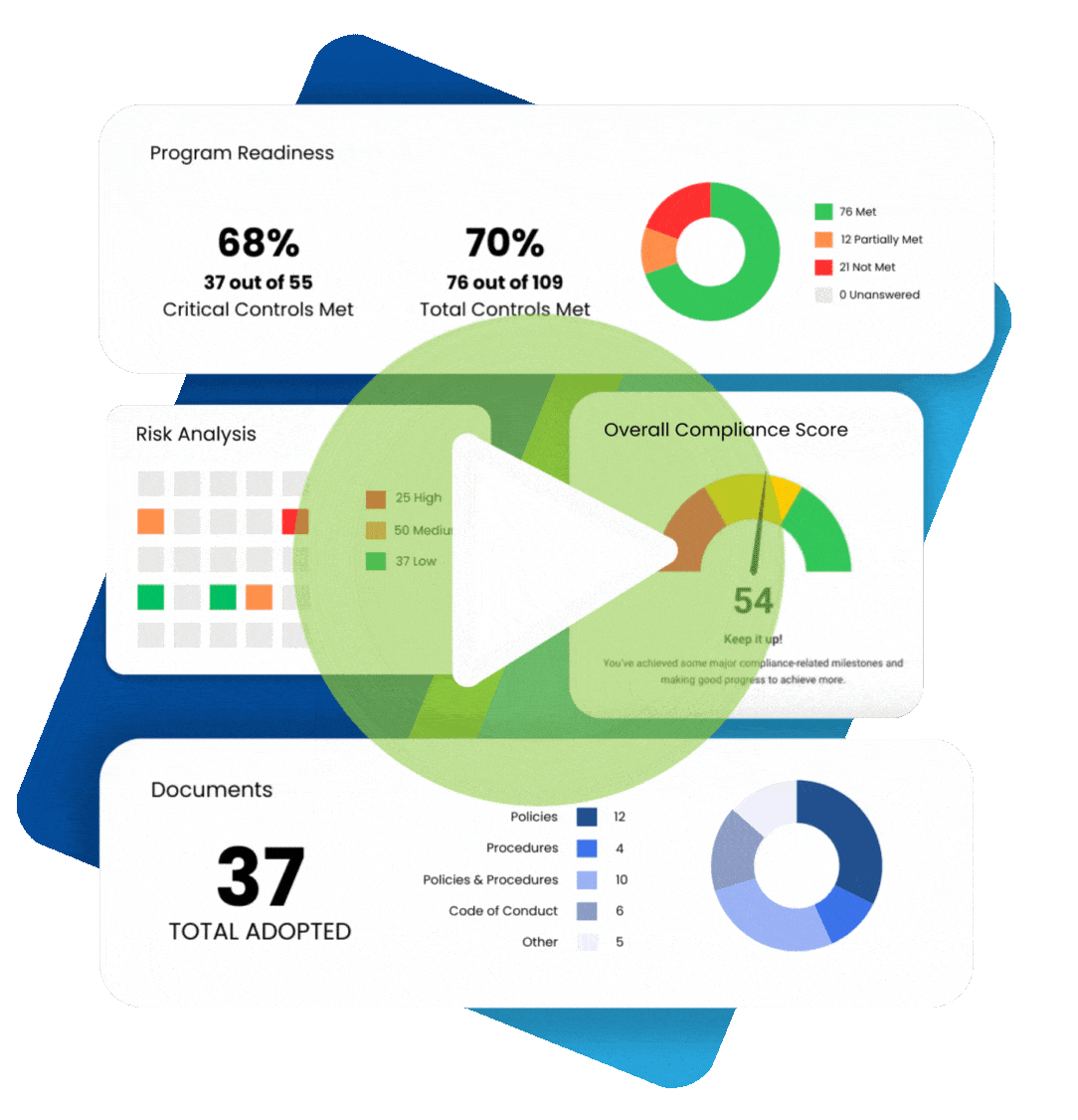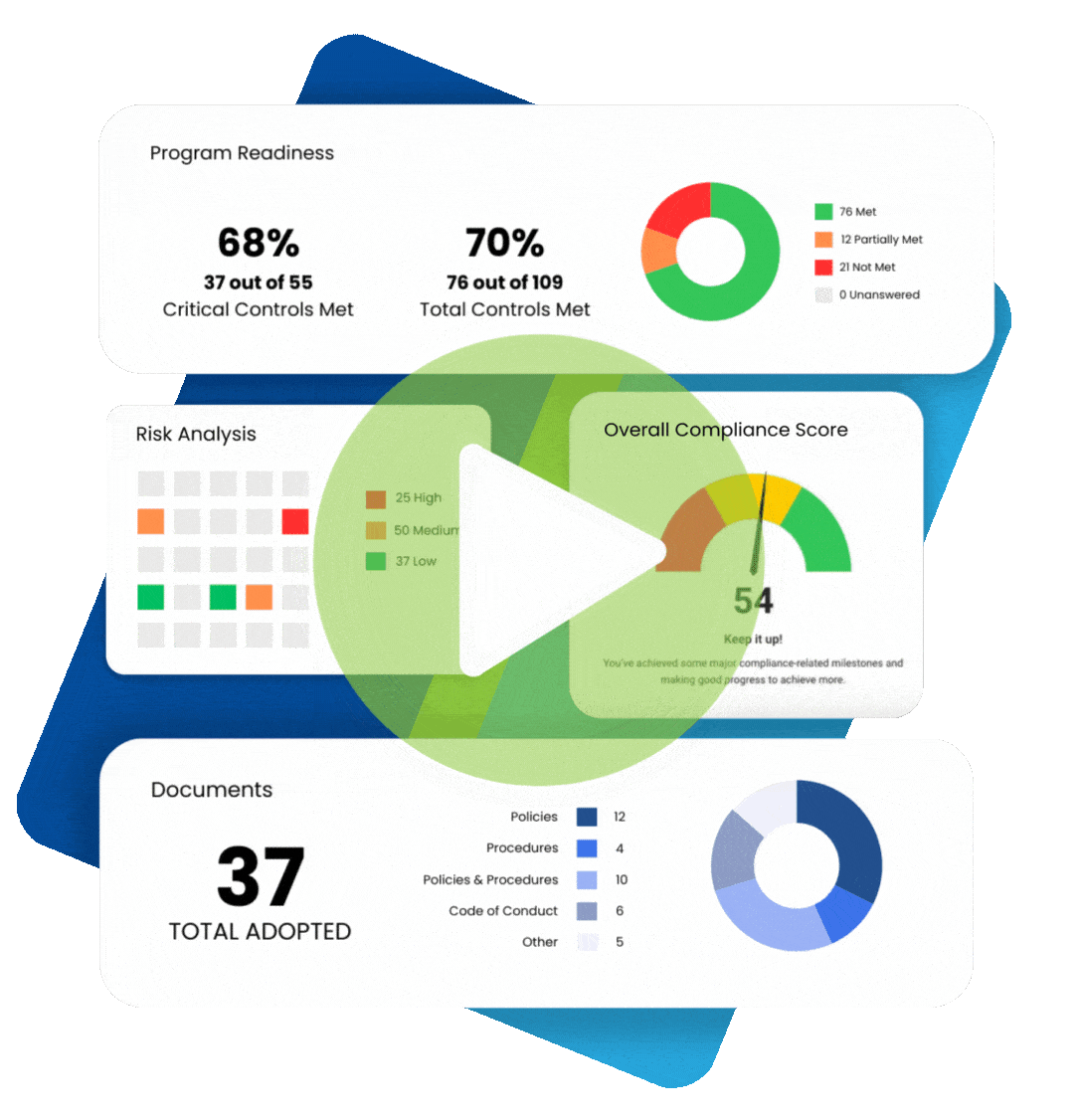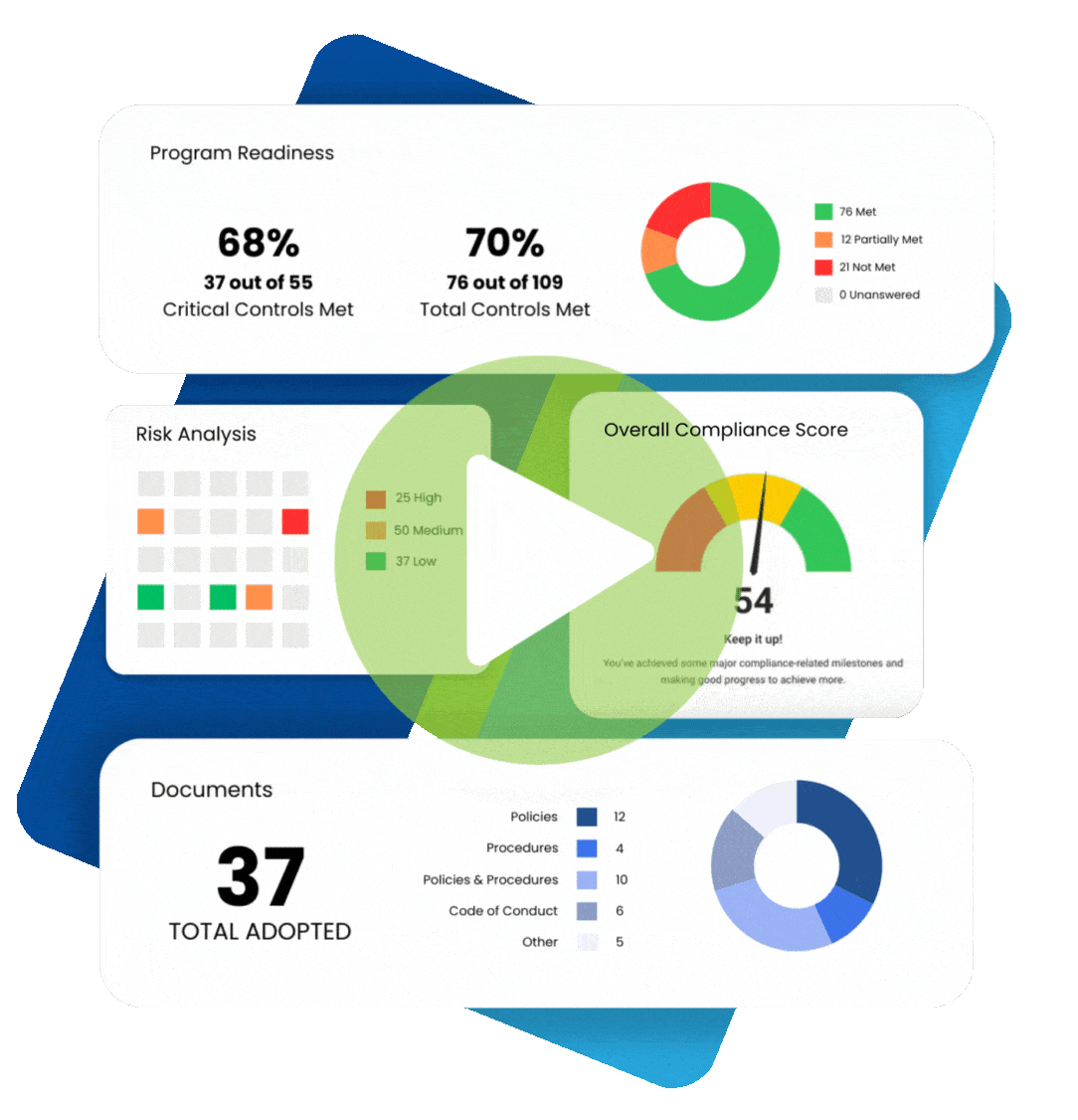
Upholding ethical standards and transparency becomes especially imperative when a healthcare organization or medical practice has been implicated in illegal or unethical practices. A Corporate Integrity Agreement allows such a healthcare entity to avoid serious legal repercussions and prevent future incidents of misconduct.
By examining the purpose and regulatory mechanisms of these agreements, we can understand their role in ensuring compliance, promoting ethical conduct, and ensuring patient and employee safety.
What Is a Corporate Integrity Agreement?
When a hospital, doctor’s office, or other healthcare organization is guilty of a regulatory or compliance violation, the U.S. Office of Inspector General (OIG) can invoke civil or criminal prosecution or licensure or other penalties, fines, exclusions from federal programs like Medicare, or revocation of billing privileges. Companies facing civil monetary penalties and exclusion from federal healthcare programs may have the option to enter a Corporate Integrity Agreement as an alternative.
The Office of Inspector General (OIG) facilitates the development of the Corporate Integrity Agreement between the U.S. Department of Health and Human Services (HHS) OIG and the organization. A Corporate Integrity Agreement is typically part of the civil settlement, and it prevents the violating entity from being added to HHS’s OIG Exclusion List, including organizations excluded from valuable programs like Medicare or Medicaid.
A Corporate Integrity Agreement outlines the healthcare entity’s future compliance obligations and the steps to prevent future fraud, abuse, and illegal activity incidents. Being able to opt into a Corporate Integrity Agreement typically depends on the severity and nature of the infraction and the entity’s previous compliance record.
In addition to Corporate Integrity Agreements for corporations, the OIG also drafts and oversees Integrity Agreements for small-group practices and individual providers. There are also OIG Quality of Care Agreements, which are relevant to infractions involving adverse effects on patient care.
What Does the Corporate Integrity Agreement Contain?
The Corporate Integrity Agreement outlines specific measures the violating organization will take over five years. These actions reflect efforts to improve and maintain compliance standards, and some of the outlined actions address the specific causes and factors contributing to the violation the entity committed. When entering an OIG Corporate Integrity Agreement, the organization agrees to
- Develop written procedures and policies for issues necessitating the Corporate Integrity Agreement
- Report overpayments, ongoing investigations and legal proceedings, and other reportable events
- Carry out a comprehensive compliance training program for all organizational members
- Hire an external compliance officer, as opposed to designating that role to a current employee
- Appoint a compliance committee that the compliance officer oversees
- Create a whistleblower program that enables confidential disclosure and reporting of compliance violations
- Verify that all existing members and new hires are not listed on the HHS OIG Exclusion List
- Submit annual Corporate Integrity Agreement implementation reports and compliance reports to OIG
- Hire an independent review organization (IRO) to conduct yearly compliance reviews
Oversight of Corporate Integrity Agreements
As previously mentioned, OIG corporate integrity agreements require oversight from IROs, which vary in size and composition depending on the requirements of the Corporate Integrity Agreement. Many companies provide IRO services, including reviews of claims, compliance measures, vetting new hires, and transactions. If the Corporate Integrity Agreement calls for oversight from specific experts, such as those familiar with Medicare or Medicaid programs, then more than one IRO may be necessary.
IROs must be external to the participating organization and act with objectivity. The Corporate Integrity Agreement outlines the qualifications for an eligible IRO, which the participating organization must identify within 30 days. After reviewing the prospective IRO, the HHS OIG approves it or directs the organization to identify a new one.
Preventing a Corporate Integrity Agreement Violation
Strict adherence to the Corporate Integrity Agreement is vital for the organization to continue participating in federal programs and avoid adverse legal consequences. Consequences of Corporate Integrity Agreement violations include:
- Increasing the original civil money penalties
- Additional monetary penalties
- Being added to the HHS OIG Exclusions List
Following the Corporate Integrity Agreement to the letter protects the organization from legal penalties and enables the participating entity to proactively uphold ethical standards, rectify mistakes and past misconduct, and maintain public trust.